![[basal ganglia inputs and outputs]](./FigV2.gif)
by Ben Best
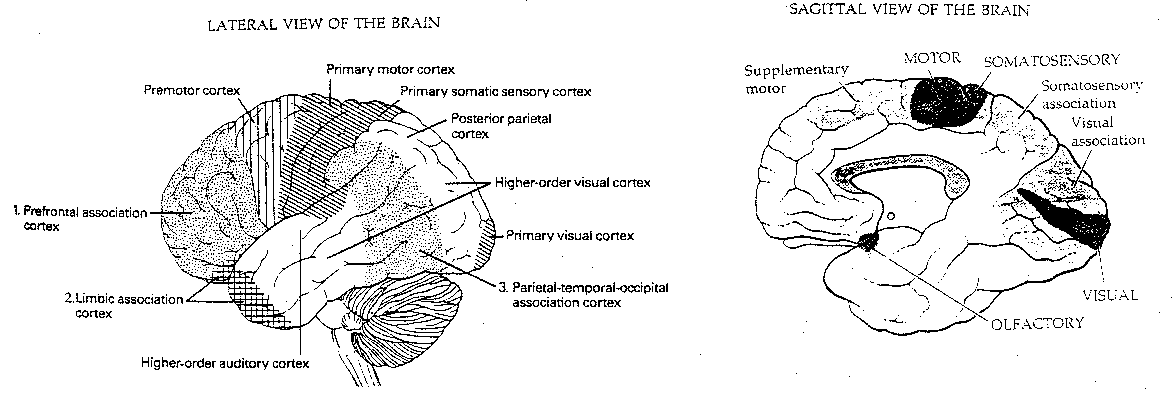
The cerebral cortex can be divided into the evolutionarily older archicortex (hippocampus and olfactory cortex) and the newer neocortex. The neocortex is not the only section of the brain which is disproportionately larger in humans and primates. So is the thalamus, the lateral lobes of the cerebellum and the hippocampus. But it is the neocortex which is commonly believed to be the organ of thinking. It is in the neocortex that demonstrably increased blood-flows occur during the performance of various mental tasks. It is therefore the neocortex that seems to be the most likely candidate for "the anatomical basis of mind".
![[the thalamus]](./FigV1.gif)
Most of the inputs of the
neocortex from outside structures
are gated through the thalamus
(from the Greek word for "bed").
The pulvinar (from the Greek
word for "pillow") receives
inputs from the midbrain and
many areas of the neocortex,
and sends outputs to many areas
of the neocortex. It seems to be
involved in eye movement
and in the integration of sensory information. The lateral geniculate body
is the relay nucleus between the optic fibers from the eye and the
primary visual cortex. The medial geniculate body is the relay nucleus
between the auditory fibers and the primary auditory cortex. The ventral
nucleus can be subdivided into three sections that relay inputs from (1) the
body (from skin, muscle and joint receptors via the medial lemniscus from
the dorsal root ganglia), (2) the cerebellum and (3) the basal ganglia — to the
somatosensory, motor and premotor cortex areas, respectively. The lateral
nucleus receives inputs from (and sends outputs to) the posterior parietal
cortex, an area associated with perception of spatial relations. The anterior
nucleus receives inputs from the mammillary body of the hypothalamus and
sends outputs to the cingulate gyrus (a region of the cerebral cortex included
in the limbic system). The mediodorsal nucleus relays inputs from the
amygdaloid nucleus, hypothalamus and olfactory area to the frontal cortical
lobes. The Y-shaped internal medullary lamina (which divides the thalamus
into lobes) contains nuclei (the intralaminar thalamic nuclei) which
receive inputs from various subcortical nuclei (including raphe nucleus,
locus coeruleus and basal nucleus) and project diffusely into various
areas of the cortex. All of the thalamic nuclei receive massive inputs from
the cortex, generally from those same areas to which they send output.
![[basal ganglia inputs and outputs]](./FigV2.gif)
Although the basal ganglia send outputs to the cortex via the thalamus, they receive inputs directly from the cortex. The receiving part of the basal ganglia is the striatum (a collective term for the putamen and the caudate nucleus) which derives its name from the slender fascicles of myelinated fibers which give it a striated (striped) appearance. The putamen receives inputs primarily from the somatosensory and motor (somatomotor) cortex, whereas the caudate nucleus receives inputs from the so-called association areas of the neocortex. The major outputs from the basal ganglia (via the thalamus) are to the supplementary motor area, the premotor area and the prefrontal cortical areas. Functionally, the basal ganglia seem to be important in the speed of initiation and execution of well-rehearsed movements. Lesions to the dopamine inputs to the striatum block the role of food and water as reward signals in procedural learning.
|
Functionally, the cerebral cortex can be divided into motor areas, primary sensory areas, secondary (higher-order) sensory areas, and association areas. The association areas are the least understood. Conversely, it may be that cortical areas which are not understood are assumed to be for "association".
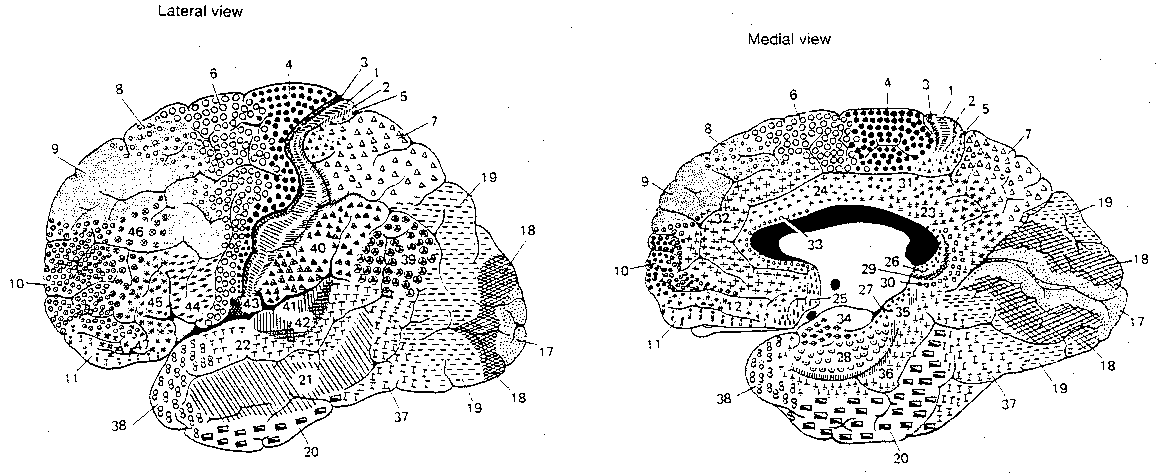
|
Of the 10 billion axons in the white matter from the cortex, only 10-20 million connect with nuclei outside the cortex. The corpus callosum, which connects the two cerebral hemispheres, contains only about 100 million axons. Thus, about 98.6% of the axons in the white matter lead from one cortical region to another within the same hemisphere.
Approximately 75-85% of the neurons in the neocortex are
pyramidal cells (pyramid-shaped), characterized by a broad base at the
bottom, and an apex that points upwards to the cortical surface. The
neurotransmitter of pyramidal neurons is glutamate, which is excitatory.
Most of the axons in the neocortex connect pyramidal neurons with
other pyramidal neurons. A large pyramidal neuron may have 20,000
synapses (the average neocortical neuron has 6,000). Non-pyramidal neurons
in the neocortex are referred to collectively as interneurons. Most of
these interneurons (smooth stellate, basket cells, chandelier
cells and double bouquet cells) use the inhibitory neurotransmitter
gamma-amino butyric acid (GABA). The other common interneuron is the
spiny stellate cell, which is excitatory. The average cortical neuron
is idle 99.8% of the time.
![[six cell layers of the cerebral cortex]](./FigV5.gif)
The gray matter on the surface of the cerebral cortex is divided into six layers. In the illustration, next to the names of the layers are three columns, indicating the results of different staining methods. The leftmost column indicates a Golgi stain, which causes silver chromate salt precipitation in less than 2% of cells — but these cells are stained in their entirety. The second column uses a stain that reveals only cell bodies and the third column uses a stain that reveals only myelin (axons).
Layer 4, often called the granular layer, contains many spiny stellate (excitatory) interneurons. It is layer 4 that receives input from the thalamus. Layer 4 tends to be thickest in primary sensory cortex and it is virtually missing in the motor cortex (the "agranular cortex"). Layer 4 is so thick and specialized in the primary visual cortex that it is subdivided into 4A, 4B and 4C. Cell density is also very high in the primary visual cortex: 250,000 neurons per square millimeter, versus 100,000 in the rest of the neocortex.
The layers above layer 4 (the supragranular layers: 2 and 3) are where communication between other cortical areas originate, whereas outputs leading outside the cortex originate in layers 5 and 6 (the infragranular layer) below layer 4.
Layer 1, the molecular layer, mainly consists of apical dendrites from pyramidal cells from lower layers — plus axons synapsing on those dendrites. It contains almost no neuron cell bodies. Layer 2 contains many small densely-packed pyramidal neurons — giving it a granular appearance. Layer 2 (the external granular layer) receives inputs from other cortical layers, and layer 4 (the internal granular layer) receives inputs from outside the cortex. Layer 3 contains medium-sized pyramidal neurons which send outputs to other cortical areas. Layer 5 contains the largest pyramidal neurons, which send outputs to the brain stem and spinal cord (the pyramidal tract). Layer 5 is particularly well-developed in the motor cortex. Layer 6 consists of pyramidal neurons and neurons with spindle-shaped cell bodies. Most cortical outputs leading to the thalamus originate in layer 6, whereas most outputs to other subcortical nuclei originate in layer 5.
Sensory inputs first activate neurons in layer 4, which propagate the excitement up to layers 2 and 3, and from there down to layers 5 and 6. Recurrent pathways will send excitation back from layer 6 to layer 4. These rich interconnections between layers, and the organization of these connections into vertical columns, have led to models of the cortex in which billions of cortical columns act as the functional units. In sensory areas, these vertically-integrated columns actually have an inhibitory effect on adjacent columns (lateral inhibition) which is believed to increase resolution of sensory information.
![[eyeball structure]](./FigV6.gif)
The receptors for vision in the eye are known as rods and cones. The photopigment of the rods is rhodopsin, composed of a protein (opsin) and retinene (an aldehyde of Vitamin A). Rhodopsin gives the retina a deep purple color, which bleaches under illumination. Three other opsins, each with a distinct photopigment, can be found in the cones — giving sensitivity to red, blue and green wavelengths. The cones need strong light, whereas the rods are much more light-sensitive and allow for night vision.
Detailed vision occurs in the central retina
of the eye, where a person fixates his or her gaze.
Corresponding to the central 1° of vision (from
the central axis) is the fovea, a pit in the
back of the retina. Corresponding to the central 5°
of vision is the macula lutea, a yellowish region
of the retina (about 2 millimeters in diameter) with
the fovea in its centre. The cones are concentrated
in the macula and provide both visual detail and color.
Cats have no macula, and therefore are color-blind
and have less visual acuity. The vast predominance
of rods outside the macula is responsible for the
fact that we can detect the motion and shape of an
object at the corner of our eye before we can detect
its color.
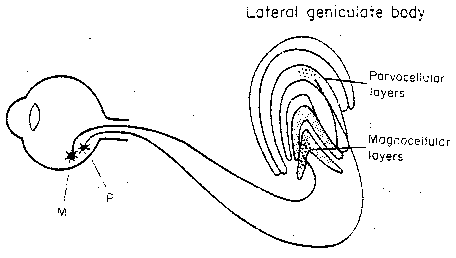
|
Ganglion cells collect input from rods and cones over
small patches of retina. These cells are primarily
M-cells (Magno = large) and P-cells
(Parvo = small). It is the small,
numerous P-cells that are responsible for
color and fine detail. The P-cells have
subtypes corresponding to different color
contrasts. Axons from the M-cells project to
the two lower layers (the magnocellular
layers) of the lateral geniculate body
(lateral geniculate nucleus, LGN).
Axons from the P-cells
project to the four upper layers (the parvocellular layers) of the LGN.
The 1.5 million neurons of the LGN then send axons to the primary visual
cortex. But the axons the LGN receives from the primary visual cortex are
more numerous than the ones it sends.
![[optic chiasm]](./FigV8.gif)
Half of the fibers in the optic nerve cross at the optic chiasm to send axons to the LGN on the opposite side of the brain. Thus, the infor- mation from the left half of each eyeball (receiving light from the right visual field) will be sent to the left LGN and onward to the left visual cortex. Conversely, information from the right half of each eyeball is relayed to the right visual cortex via the right LGN.
![[visual cortex]](./FigV9.gif)
The primary visual cortex corresponds to Brodmann's area 17 at the posterior tip of the brain. Like the striatum, it has a striped appearance and is therefore known as the striate cortex. Because of its location on the the upper and lower lips of the calcarine ("spur-shaped") sulcus, the striate cortex is also known as the calcarine cortex. Yet another name is area V1.
Like the somatosensory and the somatomotor cortex, the striate
cortex displays a topographical mapping. Shining a point of light on
the retina activates neurons in a small area of the striate cortex (and
the LGN). Moving the point of light moves the area of activated neurons
in a systematic way. Comparable to the somatomotor and the somatosensory
cortex (where the hands occupy more space than the trunk), a disproportionately
large portion of the striate cortex is devoted to the first 10 degrees around
the visual axis. Unlike other areas of the neocortex, neither hemisphere
is dominant for the striate (or visual) cortex — an important feature
for the formation of symmetric visual images in the brain.
![[visual cortex striations]](./FigV10.gif)
The striate cortex is a case study in dense
packing of cortical information — and in the
overlapping of submodalities. The striations
of the cortex (which have the appearance
of a large fingerprint) correspond to
dark regions sourced from one eye and
light regions sourced from the other eye.
![[ocular dominance columns]](./FigV11.gif) Columns of neurons within
a stripe correspond to a
region of the retina in one
eye and are therefore known
as ocular dominance columns.
Within the ocular dominance
columns can be found subcolumns
of neurons which are sensitive to
particular orientations in space
— known as orientation columns.
To complicate matters further,
color-sensitive columns known as
blobs pierce the centres of
the ocular dominance columns
resulting in interblob neurons
which are orientation-sensitive,
and blob neurons, which are not.
Moreover, blobs are specialized
on the basis of wavelength
("color") sensitivity.
Columns of neurons within
a stripe correspond to a
region of the retina in one
eye and are therefore known
as ocular dominance columns.
Within the ocular dominance
columns can be found subcolumns
of neurons which are sensitive to
particular orientations in space
— known as orientation columns.
To complicate matters further,
color-sensitive columns known as
blobs pierce the centres of
the ocular dominance columns
resulting in interblob neurons
which are orientation-sensitive,
and blob neurons, which are not.
Moreover, blobs are specialized
on the basis of wavelength
("color") sensitivity.
![[visual cortex mappings]](./FigV12.gif)
![[visual area inputs]](./FigV13.gif)
Differentiation of neuron (or
cortical column) function becomes
more orderly in the secondary
visual areas (the extrastriate
visual cortex) located in Brodmann
areas 18 and 19. To distinguish these
areas from the V1 area (striate cortex),
they are designated V2, V3, V4, V5 and V6.
V2 receives a point-to-point mapping
from V1 and, like V1, it represents
all submodalities of vision (motion,
orientation, color and depth). V3, V4,
V5 and V6 are specialized for one
submodality alone. V3, V4 and V5 have
satellite areas V3A, V4A and V5A
which mostly receive inputs from
V3, V4 and V5, respectively. Strong
inputs are shown by solid lines in
the figure,
whereas weak inputs are shown by dashed
lines. The inputs to V4 from V1 are
from the fovea alone. V6 is in a more
parietal region not shown in the diagram.
![[visual cortex orientation mappings]](./FigV14.gif)
As shown in the figure, a longitudinal penetration through
area V3A reveals neurons that have
orientation preferences in an orderly
manner. That is, by moving along the
axis, one encounters neurons that
will be activated by stimuli of
increasing angle of orientation.
Moving along a perpendicular
penetration of V3A yields
neurons that are all
activated by the same
orientation of stimulus.
![[wavelength and absorbance]](./FigV15.gif)
![[gray-scale background effects]](./FigV16.gif)
Color-perception by V1, V2 and V4 indicates some remarkable properties of the visual cortex. The three types of cones are sensitive to a range of wavelengths, achieving maximum sensitivity at about 440, 520 and 580 nanometers (corresponding to blue, green and red). Nonetheless, what we see as blue, green or red can correspond to different wavelengths depending on whether a room is lit by tungsten light, fluorescent light or daylight. The property of color constancy has survival value insofar as it assists in the identification of objects. It turns-out that neurons in V1 are actually sensitive to wavelength, whereas neurons in V4 are sensitive to color. Between V1 and V4 an automatic processing occurs to interpret each color in the context of surrounding colors and thereby "discount the illuminant". An analogous kind of processing can even occur in a black, white and grey picture. Notice that the horizontal band in the illustration looks lighter on the right than on the left. Yet it is easy to prove that the shade is uniform — by covering the background.
It was mentioned earlier that
some models of brain function
treat columns of neurons within
the neocortex as functional units
or modules. There is good
evidence for this in V2 and V5.
But it has not been demonstrated
for the whole neocortex, nor even
for V4 — despite the fact that
the visual cortex has been so
carefully studied. And the
functional complexity of V1 has
already been alluded-to.
![[Kanizsa triangle]](./FigV17.gif)
In the CRITIQUE OF PURE REASON Immanuel Kant divided the mind into the two Faculties of passive Sensibility (collection of raw sense data) and active Understanding. But many examples of "optical illusion" indicate active interpretation in the data-collection process. The distinction between wavelength and color has already been mentioned in this connection. Another example is the Kanizsa triangle in which non-existent lines are imagined to be "seen". Electrophysiological studies have shown that there are neurons in V2 which respond to these "invisible" lines.
A monkey was trained to look at colored bars without moving its eyes. Red-sensitive neurons in V4 fired more when the red bar was being rewarded and green-sensitive neurons in V4 fired more when the green bar was being rewarded. Despite the fact of identical visual input, subjective attention showed measurable differences in V4 neuronal activity, but no alteration in V1 neuron activity. Chemical inhibition of the pulvinar of the thalamus reduced the monkey's capability of shifting attention. The idea that the pulvinar controls attention to sensory stimuli is supported by studies on patients with thalamic damage and by PET scans on normal humans subjected to experimental distraction. All visual areas send fibers to the pulvinar.
Since visual attention seems closely related to "awareness" and "consciousness", this is very suggestive. One could even imagine that consciousness is centered in the pulvinar. But the same reasoning could attribute consciousness to the Reticular Activating System (RAS), since the RAS sends activating impulses to the neocortex — leading to arousal and wakefulness. But is consciousness in the source of activating impulses or in the activated neocortical neurons themselves? The latter seems more plausible. Similarly, the attention to a red or green color bar may be in the activated V4 neurons, rather than in the pulvinar (which may be the source of activating impulses). Also, one does not lose consciousness of objects in the visual field which are not the centre of attention.
The feeding of visual information from V1 into the specialized areas — V3 (orientation), V4 (color), V5 (motion) and V6 (depth) — indicates a somewhat hierarchical view of cortical processing. One might wonder whether there is yet another area in the neocortex where the information from V3, V4, V5 and V6 is brought-together into a single image. So far, no such area has been found. One might expect that if such an area existed, patients could be found with damage to the area who could not integrate the features of color, motion, etc. Moreover, the idea that such an area exists comes close to the "fallacy of the homunculus". In this view, we see, hear, touch and smell because there is a little person somewhere in our brain who sees the results of visual cortex processing, who hears the results of auditory cortex processing, etc. Yet the entire neural network view of the neocortex is that information processing is parallel and distributed. This implies that our subjective experience of the greenness of a leaf may well be identical with the activation of green-sensitive neurons in V4.
Rejecting the hierarchical view raises the question of whether V5 neurons are more "experiential" than V1 neurons, thalamus neurons or spinal cord neurons. Overall motion of an object seems to be detected by neurons in V5, while neurons in V1 detect its component parts. It seems plausible that we could grow a new spinal cord — or even a new thalamus — without compromising our identity. Monkeys in whom the V5 area is intentionally damaged have difficulty responding to visual motion, but they recover their capability within a few weeks. Does this imply that some "experiential" neurons are not essential to personal identity?
Implicit in this discussion is the implication that consciousness and experience is co-extensive with assemblies of activated neurons. This raises the question of all the emphasis placed on synapses by neural network models and by cryonicists. If synapses store memories, and thereby control which neurons are activated under certain circumstances, does that mean that there could be other features in the neurons themselves which are essential for their state of activation (and, therefore, for their quality of conscious experience)? Some neurons may send only one impulse down their axon upon depolarization, whereas others fire repeatedly — the large pyramidal neurons in layer 5, in particular. One researcher has suggested that "The frequency of neural impulses codes subjective certainty: a high impulse frequency in a given neuron corresponds to a high confidence that the percept is present in the external world." But is the impulse frequency and quantity a stored (learned) property of the neuron, or is it an intrinsic property of neuron type and size?
If V1 neurons respond to wavelength and V4 neurons respond to color, V4 neurons would seem more likely to be "experiential", since we experience color rather than wavelength. In the fusiform gyrus, neighboring V4, is an area which is critical for recognizing familiar faces. Patients with damage to neurons in this area suffer from prosopagnosia — they cannot identify persons by looking at faces, despite the fact that the see the faces and can recognize facial expression (like smiling, anger or startlement). Nonetheless, lie detectors indicate that the patient's brain somehow does recognize faces, even if the patient is not aware of the recognition. If this is true, where is the recognition occurring, if not in the damaged area? Could it be that recognition-of-face neurons and "experiential" neurons of facial recognition are distinct and not adjacent?