by Ben Best
To some people, preservation of memory is the most essential task of cryonics, whereas others regard feeling as being more critical. I am somewhat skeptical of both these views, but I do not have an alternative thesis — I am searching for one. If memory is critical to identity, why do I perceive that in the last year I have added memories, but not altered my identity? If some memories are more critical for identity than others, what are those critical memories and where do they reside? It may be true that to abolish all my memories would abolish my identity — but it is also true that stopping my heart abolishes my identity. That does not prove that my heart is the essence of my identity. Of course, I would prefer cryonic procedures that preserved all of my memories. My identity may remain intact if I lose my vision or a year's worth of memories, but I prefer to keep my vision and my memories — along with my identity.
Our neurological hardware consists of plastic and non-plastic components. If the essence of our identities lie in the non-plastic components, then preservation of memory may be neither necessary nor sufficient to preserve our identities. There is some plasticity to the neurological circuits governing vision and walking, but the neurological wiring is predominantly not plastic. Moreover, hard-wiring of our nervous system may be reflected not only in our sensory and motor apparatus, but in our behavior and thought. It is essential that neurological control of heartbeat and respiration be hard-wired. Might the perception of self and the drive for self-preservation be equally essential — and require hard-wiring?
Spiders can weave intricate spiderwebs, but this complex behavior is not learned — it is built-in neurological machinery. A female bird that is hatched and reared in isolation from other birds is still capable of building a perfect nest.
Even when learning does occur, neurological wiring may dictate which experiences result in learning and which do not. Many birds learn to form a strong emotional bonding at birth to any nearby distinctive and animate object — a process known as imprinting. Many animals develop strong aversion to a tasty food following a single experience of nausea after eating it. A painful stimulus following food consumption does not produce "food aversion" — nor does pairing a visual or auditory stimulus with nausea result in aversion to those stimuli.
Many human behaviors are the result of complex neurological hard-wiring. These include swallowing, coughing, orgasm, yawning, sneezing, vomiting and even laughing. Facial expressions of emotions such as anger, fear, disgust and joy are recognizable among all humans and are probably hard-wired, although subject to modification and conscious control. The linguist Naom Chomsky has even proposed — based on his perception of common principles of grammar in human languages — that the structure of language is determined by the structure of the brain.
Experience is more likely to result in learning when it has relevance to the life of the organism — especially when connected to pleasure or pain, fear or satisfaction. Some learning occurs only after repeated trials, whereas other learning results from a single experience. Learning is the process of acquiring knowledge, whereas memory is the retention of knowledge. It may be that preserving memory is critical for preserving identity, but that preserving the capacity for learning is not critical. Nonetheless, understanding the mechanisms by which knowledge is acquired may be the key to understanding how knowledge is stored.
This technical analysis of the mechanisms of learning must begin with descriptions of the simplest kinds of learning in very simple animals — along with descriptions of observable physiological changes that accompany learning. But to make these physiological descriptions comprehensible to the layperson, I must first review some fundamentals of cell biology and synapse physiology.
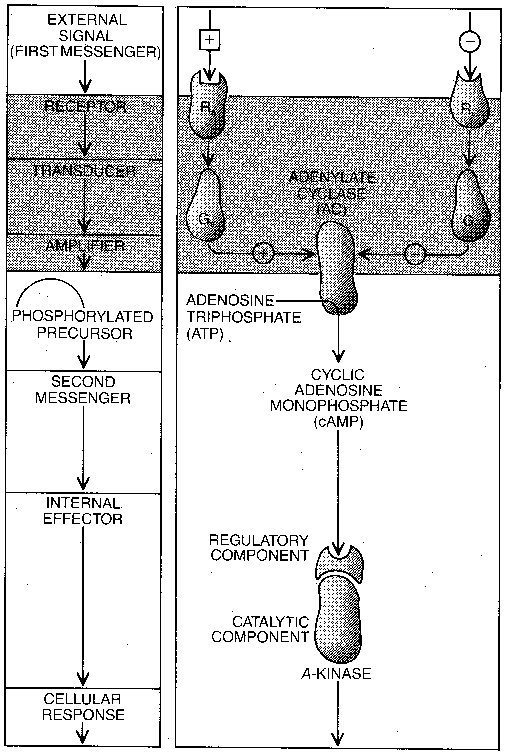
The term second messenger refers to a molecule in a cell that communicates information or change throughout the cell. A first messenger would be a molecule (usually) that communicates information or change from one cell or cell group to another, like a hormone or neurotransmitter. First messengers are outside the cell, whereas second messengers are inside the cell. First messengers attach themselves to receptors on the outside of cell membranes and begin a "cascade" of events that lead to the release of second messengers inside of cells.
The second messenger cyclic Adenosine MonoPhosphate (cAMP) is
formed from Adenosine TriPhosphate (ATP) by the enzyme adenylate cyclase.
ATP is the molecule that provides cells with the energy they require to
function. Adenylate cyclase serves to amplify the signal from the first
messenger, because once activated it can convert many ATP molecules into
cAMP second-messenger molecules. The other prominent second messengers are
cyclic Guanosine TriPhosphate (cGTP), inositol triphosphate,
DiAcylGlycerol (DAG) and calcium ion. cGMP acts as a second messenger
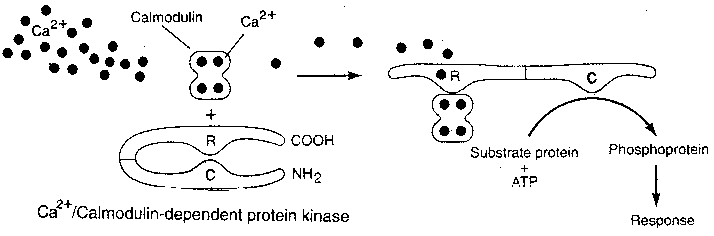
in the retina and in the Purkinje cells of the cerebellum. Calcium usually
acts as a second messenger when its ion is attached to the protein
calmodulin.

|
Second messengers exert
their effects directly and
indirectly by attaching them-
selves to proteins and causing
a conformational change. An
example of a direct effect is
the protein conformational
change due to calcium in muscle which leads to
muscle contraction. More commonly, second
messengers act indirectly by attaching themselves
|
|
Protein kinases activated by second
messengers cause reabsorption of water in
the kidney, secretion of digestive enzyme
by the pancreas, breakdown of lipids in
fat cells, fluid secretion in the intestine
and the opening of ion channels in neurons.
Protein kinases also induce long-term
changes by altering DNA synthesis. For
example, antigens act as first messengers
to lymphocytes — activating a second-
messenger system that leads to protein
kinase action on DNA proteins resulting
in the production of antibodies specific
to the antigen. Normal cell growth is
determined by first messengers known as
growth factors which alter cellular
DNA through second messenger systems to
cause cell enlargement and cell division.
The activity of protein kinase on the DNA
of neurons is the key to structural changes
in the nervous system that are associated
with long-term memory.
|
When the first messenger attaches to the receptor on the cell membrane, it exerts its effect through a G-protein, ie, a protein which is activated by the attachment of Guanosine TriPhosphate (GTP). When a first messenger attaches to the receptor, it allows GTP to attach to the G-protein. With GTP attached to it, the G-protein can activate the adenylate cyclase to convert many cellular ATP molecules to second-messenger cAMP. The receptor, the G-protein and the adenylate cyclase are all attached to the cell wall membrane. Eventually, a GTPase enzyme will convert the GTP attached to the G-protein to GDP (Guanosine DiPhosphate), thereby stopping the activity of the adenylate cyclase. (GDP remains bound to the G-protein.) The toxin produced by cholera bacteria alters G-protein in the intestine cells so that it cannot be inactivated by GTPase, thereby allowing unlimited production of cAMP second-messenger by adenylate cyclase. The result is continuous fluid secretion in the intestine, leading to diarrhea. Wasp venom acts like GTPase, accelerating the conversion of GTP to GDP and thereby stopping adenylate cyclase activity.
I have attempted to explain second-messenger systems in such excruciating detail because understanding these systems is central to understanding the cellular biology of learning and memory. I can appreciate the fact that these long sequences of molecular events are not intrinsically interesting, but I feel that the risk of being boring and tedious is far outweighed by the benefit of understanding these mechanisms. Second messenger systems are found throughout the body, but they are of particular interest in the synapse of neurons.
|
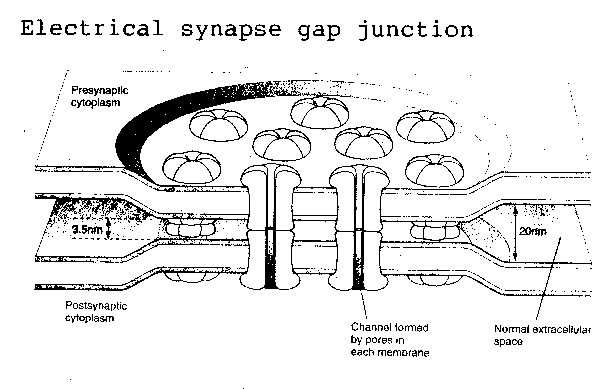
The human brain contains at least 100 billion neurons and 100 trillion synapses — which means that the average brain neuron makes about 1,000 synapses. Synapses can be either electrical or chemical. Electrical synapses are referred-to as "gap" junctions, and are not commonly found in the human brain, although gap junctions do connect heart muscle cells. Essentially they are ion channels running through one cell membrane to the cell membrane of an adjoining cell. Gap junctions allow for speed of transmission and bidirectional flow of ions between cells, but do not exhibit plasticity or other properties which give synapses a central role in learning and memory.
Chemical synapses, by contrast, can act like diodes, rectifying a signal to travel in one direction only. A depolarized axon can send an action potential either toward or away-from a cell body, but a synapse can only permit a neurotransmitter to cross from a presynaptic membrane to a postsynaptic membrane on an adjoining neuron (or back to the presynaptic membrane).
Chemical synapses can be excitatory or inhibitory. Whether a synapse is excitatory or inhibitory depends entirely on the receptors on the postsynaptic membrane, not on the presynaptic membrane or the chemical released at the synapse (apart from the fact that the receptor is built to detect a certain transmitter). An excitatory receptor results in an Excitatory PostSynaptic Potential (EPSP) and drives the postsynaptic neuron closer to the depolarization threshold which makes the cell "fire" an action potential. An inhibitory receptor results in an Inhibitory PostSynaptic Potential (IPSP) and drives the postsynaptic neuron further from the depolarization threshold. The neurotransmitter acetylcholine is excitatory at so-called nicotinic receptors, such as are found at the junction between motor neuron synapses and muscle cells (the neuromuscular junction). But acetylcholine is inhibitory at so-called muscarinic receptors, such as are found at the junction of the vagus nerve and the heart.
In the brain, the receptors for glutamate are typically excitatory,
whereas the receptors for Gamma AminoButyric Acid (GABA) are typically
inhibitory. The postsynaptic membrane of a neuron often has receptors for
both glutamate and GABA. Excluding neuropeptides, any given neuron will
release only a single neurotransmitter. Thus, axon terminals from many neurons
can connect to a given neuron and release a variety of neurotransmitters
which impinge on excitatory and inhibitory receptors to produce EPSPs and
IPSPs. The postsynaptic neuron acts like a tiny computer, summing the
EPSPs and IPSPs which determine whether or not it will "fire".
|
Even without the IPSPs, the firing of a brain neuron is a complex event. A single impulse to a neuromuscular junction is generally adequate to cause contraction of the postsynaptic muscle cell. But in the spinal cord, the EPSP will typically be 0.2-0.4 milliVolts in amplitude, as compared to the 10mV depolarization threshold the postsynaptic motor neuron requires to fire. Thus, inputs from at least 75 afferent neurons are required to produce firing. This picture is further complicated by variation in time constant between neurons — some cells can experience their EPSPs over a wider period of time than others. Moreover, the length constant of a neuron will determine how physically close presynaptic terminals must be before an action potential is generated in the postsynaptic neuron. Thus, chemical synapses make for a signalling complexity that is many orders of magnitude greater than the 100 trillion synapses alone would imply. A cryonicist could easily despair of ever preserving such a complex system. But it is important to remember that many changes have occurred to our synaptic networks over the course of our lives, and in the process of accumulating memories. Stroke victims have lost relatively large areas of their brains without losing their identity.
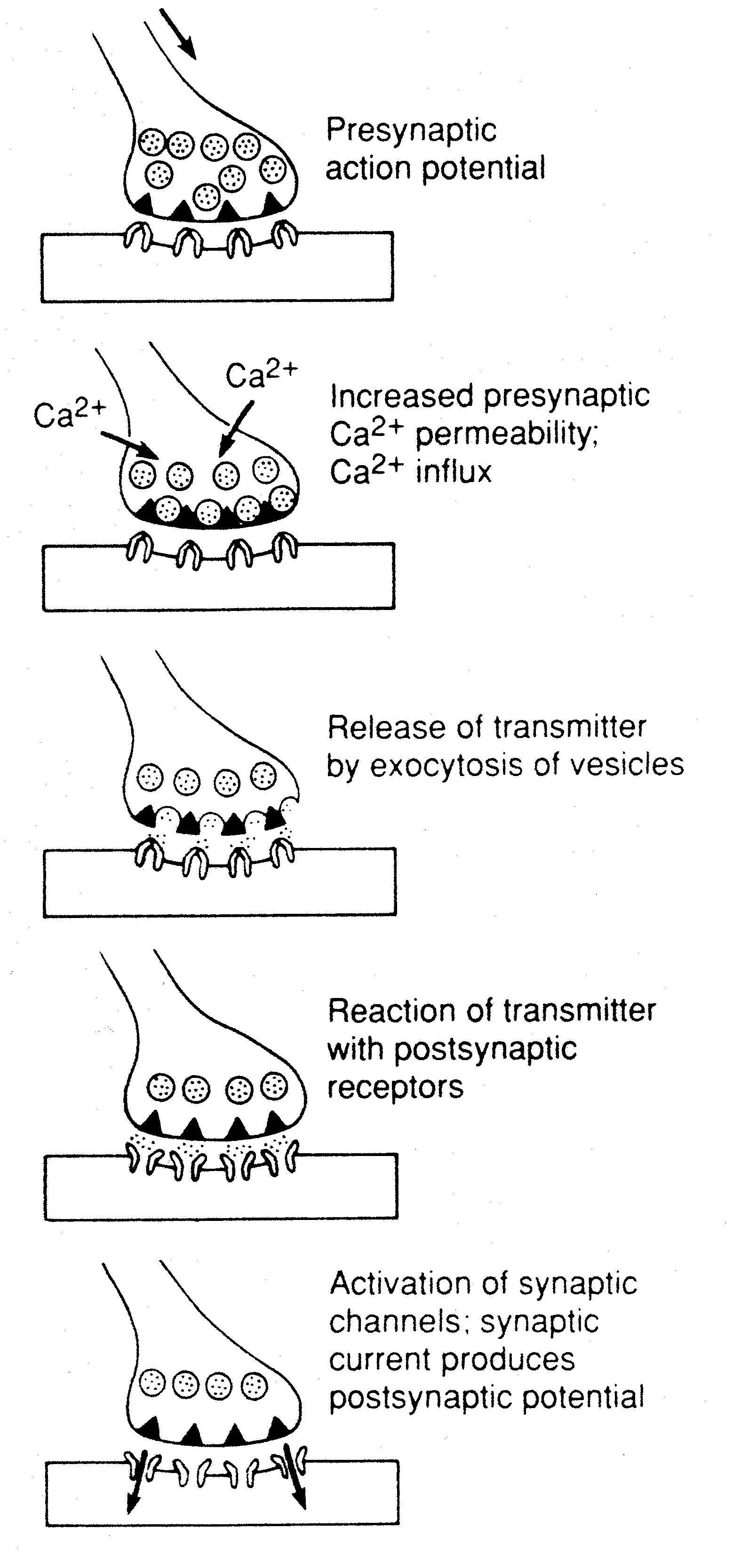
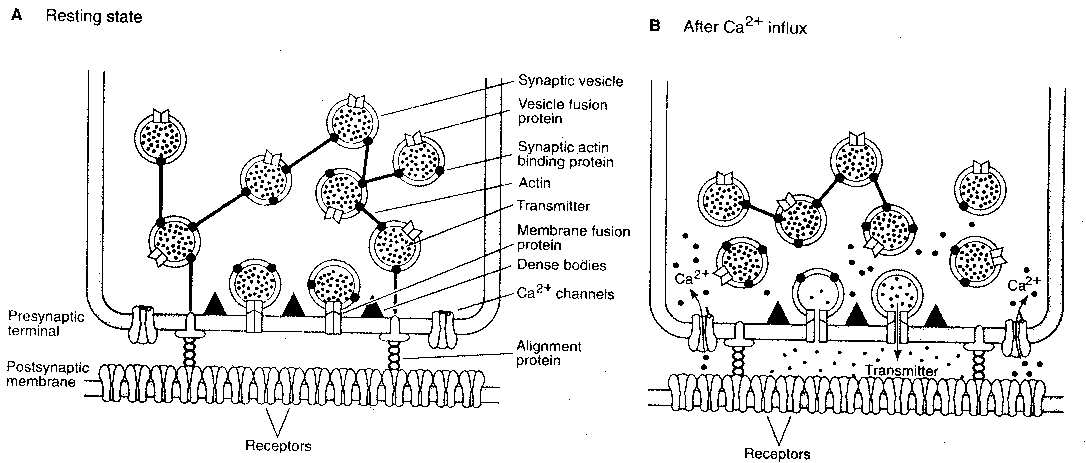
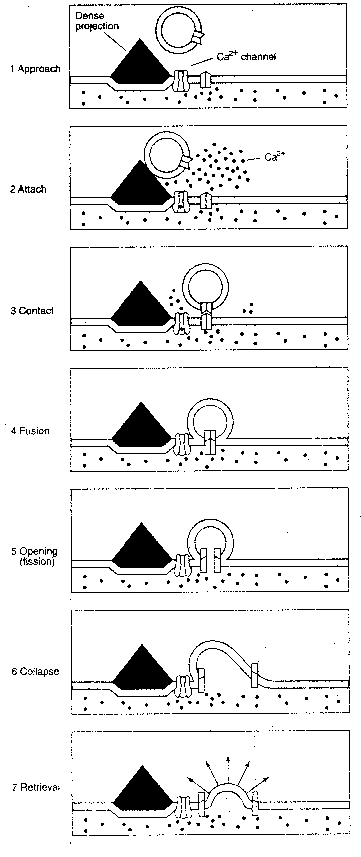
The sequence of events in the presynaptic
terminal leading to transmitter release is
the arrival of a presynaptic action potential
which opens calcium ion channels, allowing
calcium ions (Ca2+) to flow into the terminal.
(The calcium ion concentration in the
extracellular space is over 10,000 times as
great as it is in the presynaptic cytoplasm.)
The calcium ions cause vesicles containing
neurotransmitter to fuse with the presynaptic
membrane and release neurotransmitter into the
synaptic cleft. The vesicles are typically bound
together in a network connected by a protein named
actin, which is dissolved by calcium ions.
Moreover, vesicles tend not to be distributed
randomly along the presynaptic membrane, but are
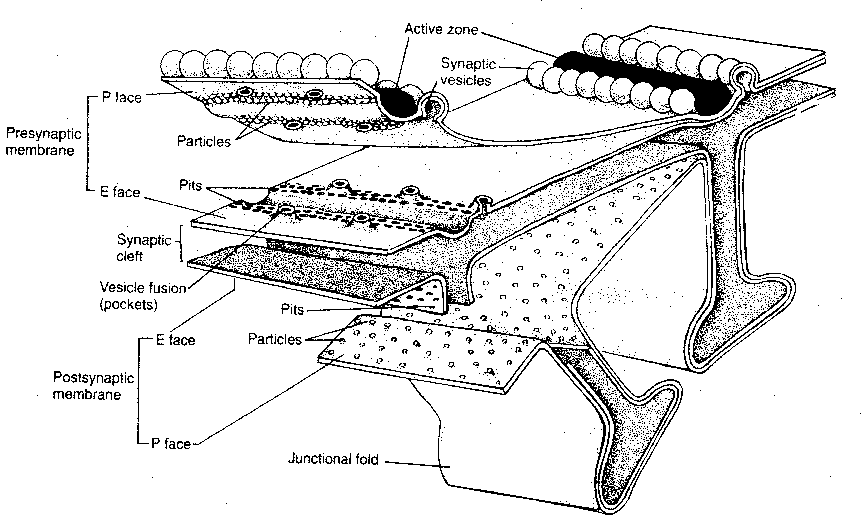
concentrated into specialized regions known as
active zones. These active zones contain dense
projections for the attachment of neurotransmitter
vesicles. It is known that calcium channels tend
to be concentrated near the active zones. Rows
of particles on either side of the dense projections,
as seen in electron micrographs, are thought to be
calcium channels. Calcium ion influx
is ten times greater at the active
zones than it is elsewhere in the
terminal.
|
|
|
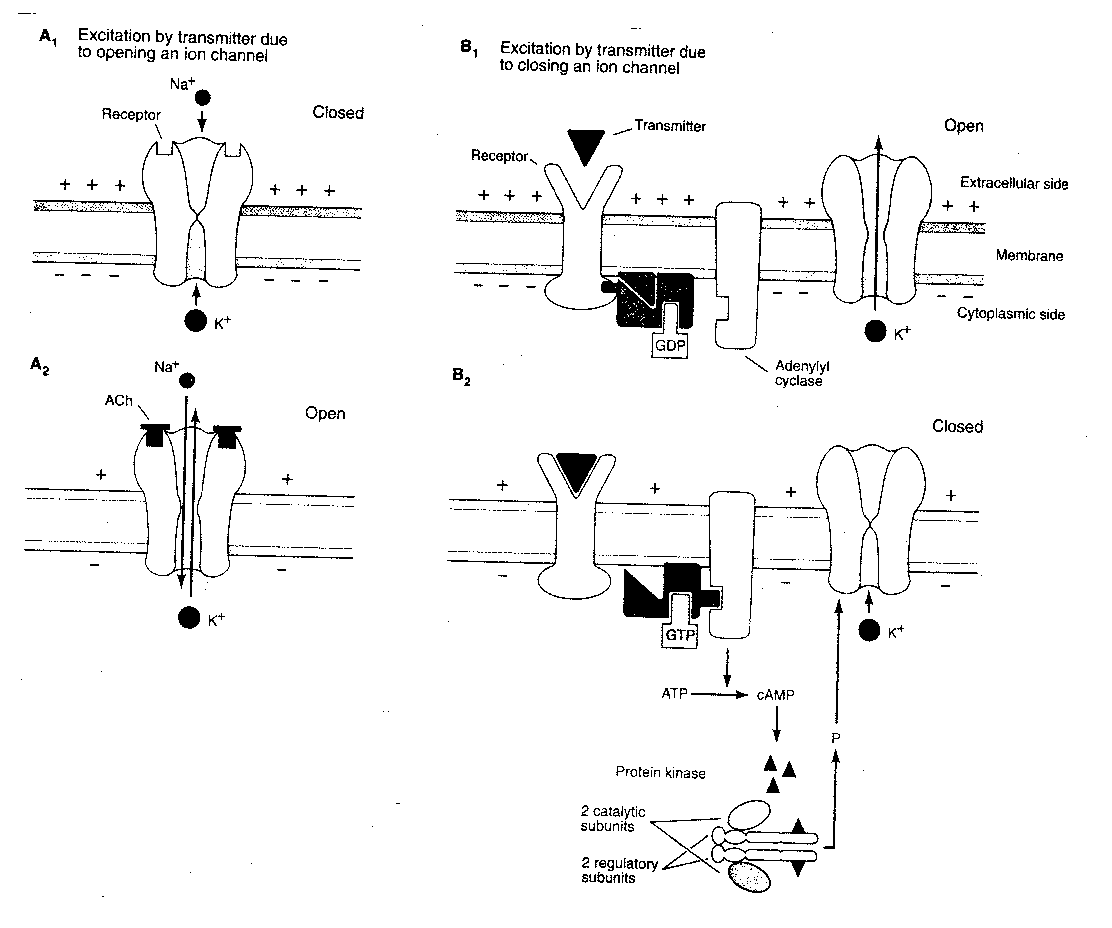
Ion channels on the postsynaptic
membrane can be classified as
directly-gated or indirectly-gated.
Directly-gated ion channels
are directly connected to
neurotransmitter receptors which
control whether they are open
or closed. Indirectly-gated ion channels are
not connected to receptors on the cell
membrane, but are controlled through second
messengers. Nicotinic receptors for acetylcholine at the neuromuscular junction
control directly-gated ion channels, whereas muscarinic acetylcholine
receptors on the heart control indirectly-gated ion channels. The
neurotransmitters GABA and glycine tend to have direct-gating receptors,
whereas noradrenaline, dopamine and serotonin tend to have indirect-gating
receptors. One glutamate receptor, the NMDA receptor, controls its
ion channel through both direct and indirect gating.
|
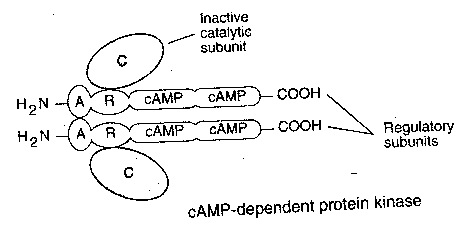
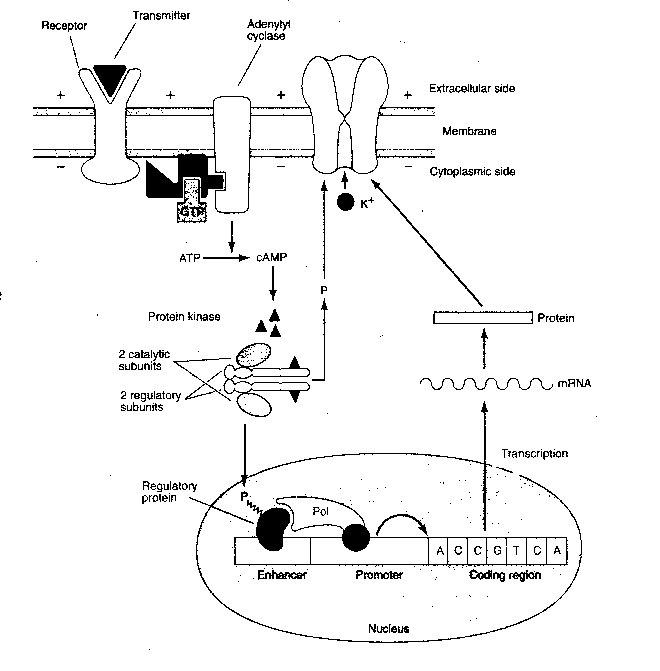
For a typical indirectly-gated ion channel, a neurotransmitter will attach itself to a receptor, causing a G-protein to exchange GDP for GTP. The GTP-containing G-protein then activates adenyl cyclase to convert ATP to second-messenger cAMP. cAMP then binds to the regulatory units of cAMP protein kinase, thereby releasing the catalytic units to phosphorylate the ion channel — which opens and allows potassium ions (K+) to exit. Although second messenger systems are slower and seem unnecessarily complicated, they have several advantages over direct gating. Indirect gating allows for considerable amplification of the input signal. Amplification can occur at each step of the "cascade". G-protein activated adenylate cyclase generally produces many cAMP second messengers before being inactivated. Moreover, second messengers can induce changes throughout the postsynaptic neuron cytoplasm, not simply at the ion channel. Second messenger systems produce the structural alterations which are necessary for long-term memory.
Not only can the catalytic
subunits of protein kinase
phosphorylate the ion channels, but
they can enter the cell nucleus and
phosphorylate transcriptional
activator proteins that bind to a specific regulatory
region of DNA (called the cAMP-Response Element, or CRE). Transcription
of DNA results in messenger RNA (mRNA) that enters the cytoplasm to direct
the manufacture of proteins. These proteins may make more lasting alterations
to the ion channels or produce other structural changes throughout the cell.
|
|
The cellular physiology of learning
and memory is known in the greatest detail
for the sea slug Aplysia californica.
Aplysia has about 20,000 neurons in the
nervous system consisting of nine ganglia
— four pairs of symmetrical ganglia and
one large abdominal ganglion consisting
of two lobes (misrepresented in the
illustration).
|
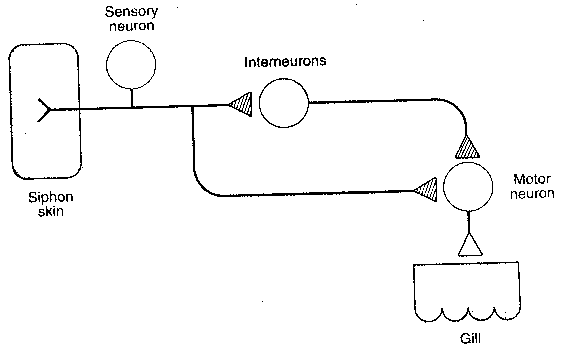
Perhaps the simplest form of learning occurs when an organism learns to ignore an innocuous stimulus, a form of learning known as habituation. If a soft jet of water is applied to the siphon of Aplysia, it withdraws its gill. With repeated jets of water to the siphon, Aplysia soon greatly reduces the extent to which it withdraws its gill. A fairly simple "wiring diagram" shows the neurons involved in this phenomenon. Experiment has shown that habituation occurs at the synapse of the axon originating from the sensory neuron and terminating at the motor neuron. Habituation can be short-term or long-term. The application of 10 siphon stimulations at one minute intervals leads to habituation that lasts no more than a couple of hours. This is short-term habituation. The application of 10 siphon stimulations at one minute intervals on 4 consecutive days leads to habituation that lasts for 3 weeks. This is long-term habituation. Forty applications on a single day does not result in long-term habituation — no habituation is observed within one day following the applications.
Short-term habituation is correlated with two observable physiological changes in the presynaptic terminal: (1) less calcium ion enters the cell upon stimulation after short-term habituation. This is thought to be due to a decreased activation of a calcium-channel. (2) only 11% of neurotransmitter vesicles are within 30 nanometers of the presynaptic active zone membrane in short-term habituation, as opposed to 28% in normal terminals.
Long-term habituation shows more
significant changes: (1) 10% of terminals
have active zones as opposed to 40% in
normals (2) the mean area of active zones
is smaller (3) the number of vesicles
within 30 nanometers of the presynaptic
membrane is smaller and (4) the total
number of synapes per neuron is smaller.
|
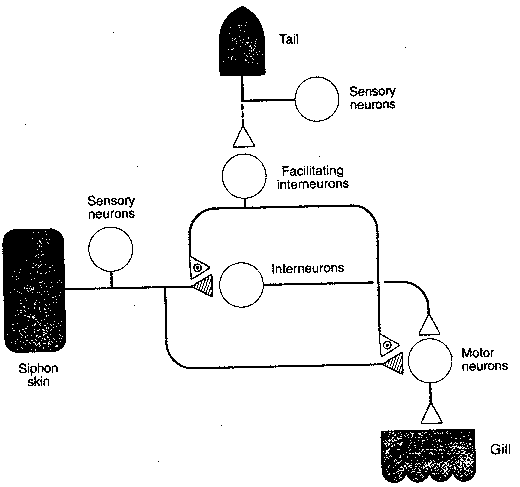
Sensitization is a more complex
form of learning than habituation. A rough
analogy might be being alone in a large house
at night and suddenly hearing a loud crash.
Immediately following the crash you would
probably become acutely sensitive to
subsequent sounds, sights, odors or tactile
stimuli. In Aplysia the sensitizing stimulus
is a strong shock to the tail. Following the
shock, a soft jet of water applied to the siphon
results in a stronger-than-normal withdrawal
of the gill. The "wiring diagram" shows the
relevant neurons and connections. As with
habituation, all the physiological changes
occur at the synapse of the axon originating
from the sensory neuron and terminating at the
motor neuron. The critical difference
is that a facilitating interneuron
forms an axo-axonic synapse on the
presynaptic membrane. The sensitizing
stimulus causes the facilitating
interneuron to release serotonin
(5-HydroxyTryptamine, 5-HT) at the
axo-axonic synapse. The presynaptic
membrane of the sensory-motor synapse
has serotonin receptors connected to
second messenger systems. As with
habituation, short-term and
long-term sensitization can
be observed.
|
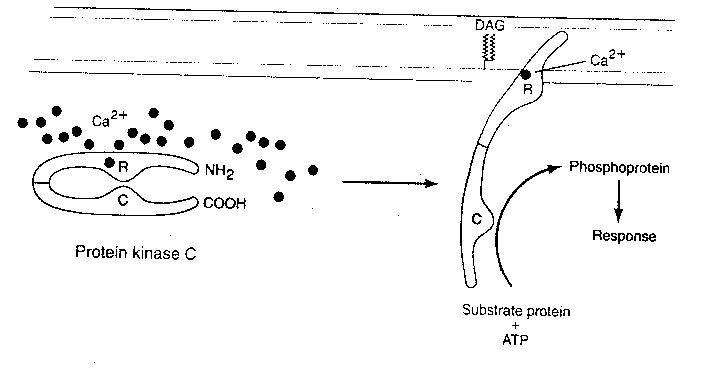
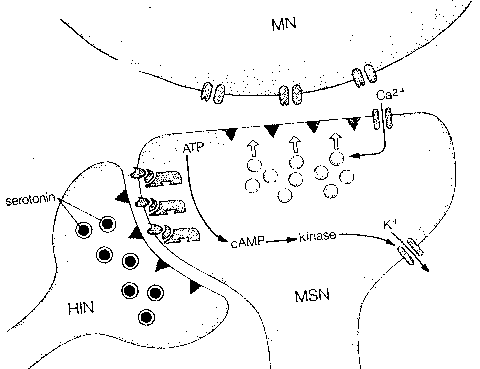
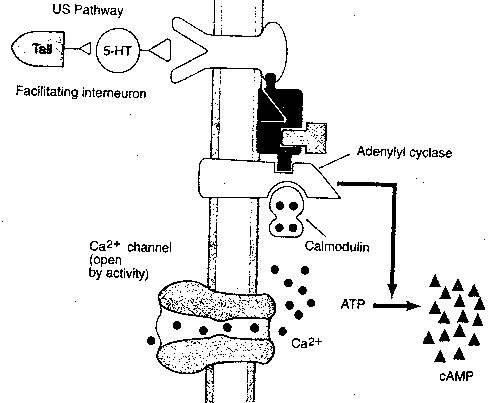
Short-term sensitization is correlated with three observable physiological changes: (1) a cAMP second-messenger cascade leads to phosphorylation of a potassium ion (K+) channel — closing the channel. Reduced K+ outflow from the cell prolongs the action potential and allows more calcium to enter (2) serotonin and cAMP directly increase the number of transmitter vesicles within 30 nanometers of the presynaptic membrane and (3) serotonin and cAMP directly influence another type of calcium channel to allow more Ca2+ into the cell. Protein Kinase C (PKC) is also activated by serotonin.
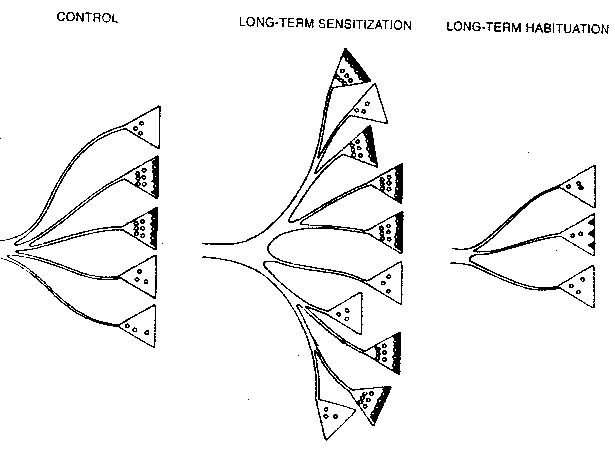
Long-term sensitization doubles the number of synaptic terminals
and increases the proportion of terminals with active zones from 40% to 65%.
Catalytic units of cAMP-dependent protein kinase activates nuclear genes
to produce two classes of proteins. One class led to the growth of new
synapses. The other protein is an enzyme which disables kinase regulatory
subunits, allowing for more persistent kinase activity.
|
Aplysia has also been subjected to a form of classical conditioning. In Pavlov's classical experiments with dogs, a bell was rung immediately prior to presenting the dogs with meat powder. The dogs learned to salivate upon hearing the bell ring. In Aplysia a shock to the tail follows a soft jet of water applied to the siphon so that Aplysia "learns" to expect a shock after a siphon stimulus, and withdraws its gill accordingly. The stimuli are identical to those seen with sensitization, except that the order of application is reversed. Moreover, the physiological responses are very similar to those seen in sensitization. Critics have noted this and denied that Aplysia is demonstrating classical conditioning. The fact that stimulating the siphon results in gill withdrawal in an untrained animal makes it seem less of a novel stimulus for the response than ringing a bell to produce salivation. Defenders claim that all responses are pre-wired, and that classical conditioning only unmasks a subthreshold connection causing dogs to salivate in response to a bell.
Nonetheless, Aplysia does exhibit
what appears to be conditioning in the
form of a more prolonged gill withdrawal
in response to soft stimulation of its
siphon. Moreover, a distinct physiological
phenomenon is seen: the arrival of the
action potential from the siphon stimulation
allows calcium ion into the terminal prior
to the binding of serotonin due to the
tail-shock. Calcium causes calmodulin to
bind to adenyl cyclase in conjunction
with the G-protein. This causes the adenyl
cyclase to produce more cAMP second
messenger than would have been produced
without the calmodulin.
|
Habituation has been demonstrated in spinal cord preparations of frogs and cats, but the presence of interneurons and other technical difficulties have thus far presented insuperable barriers to the elucidation of cellular physiology. Goldfish trained to avoid an electric shock by pairing the shock with light have shown that protein synthesis is essential for establishing long-term memory, but not for short-term memory.
Considering that the structure and function of Aplysia neurons
are so similar to vertebrate neurons, it seems quite plausible that memory
is stored by increasing synapses, increasing active zones and increasing
transmitter vesicles at selected neurons in the brain — although we must
await definitive proof. Assuming that memory is stored by this means, it seems
unlikely that cryonics
procedures could be perfected well-enough to preserve
short-term memory, but avoiding structural damage to synapses and the active
zones of synapses may be adequate to preserve long-term memory. It is
probably even more important to preserve the state of the transcription
regulator proteins in the neuron nucleus — such preservation may be
adequate for reconstruction even if the synapses and active zones are lost.
It may be even more difficult to reconstruct the state of the transcription
regulator proteins from the number of synapses and active zones. The
"redundancy" of information may be very valuable where there is partial
destruction of both the transcription regulator proteins and the synapses.
Most of what is known about the basis of learning and memory in the vertebrate brain involves correlating activity of certain types of neuron groups with certain types of learning. Details of cell physiology are rarely obtainable by current methods.
There are evidently many different areas in the human nervous
system where learning occurs — and many means by which learning occurs.
Preserving many or even any of these mechanisms may not be essential to
the preservation of identity — or even to the preservation of memory.
Preserving long-term habituation of spinal
reflexes does not seem crucial for
maintaining identity.
|
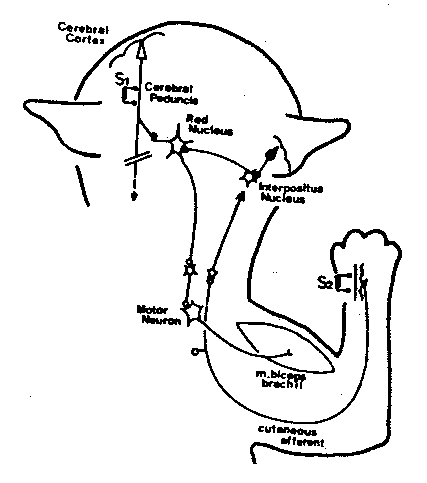
The cerebellum seems to be important in learning certain types of motor skills, especially those involving balance and co-ordination — like riding a bicycle. Cats have been trained to associate a tone with an electric shock to the forearm — to withdraw the forearm upon hearing the tone. The interpositus nucleus of the cerebellum and the red nucleus of the midbrain are the critical intermediaries in a circuit linking the cerebellum to the cerebral cortex in this classical conditioning experiment. New synapses are apparently formed in the red nucleus in association with this learning.
Rats raised in an environment where they could
exercise and be active showed 23% more spines on the
dendrites of Purkinje cells of the cerebellum
than rats in cages allowing only enough space
for access to food and water. Similarly, rats raised
in an "enriched" environment allowing for
exploration showed increased branching of
dendrites in the primary visual area
of the cerebral cortex.
|
Operant conditioning is similar to
classical conditioning, except that learning
occurs through reward or punishment of
spontaneous behaviors, rather than through
the induction of conditioned reflexes to
novel stimuli. A laboratory animal that
learns to press a lever to get a food pellet
is demonstrating operant conditioning. The basal
ganglia are evidently very important in learning
that links rewards with motor activity.
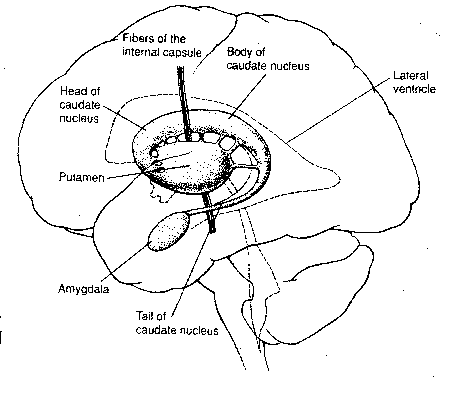
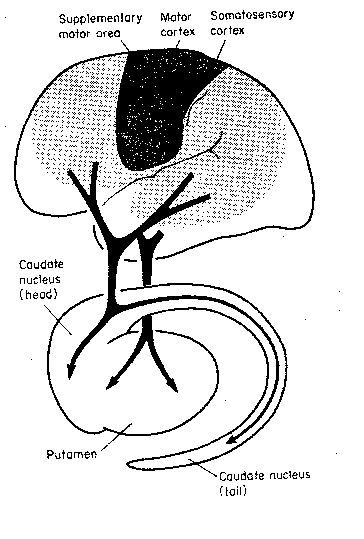
The caudate nucleus and the putamen
are basal ganglia that receive inputs primarily
from the cerebral cortex — the caudate nucleus
from the association areas and the putamen from
the somatosensory and somatomotor gyri. These
two nuclei are collectively called the striatum.
|
Operant conditioning, however, has been
demonstrated on a single neuron of the somatomotor
cortex. A microelectrode was implanted on a
single motor neuron of a monkey, and the monkey
was rewarded with drops of fruit juice when the
firing of that neuron exceeded a preset level.
With conditioning, bursts of impulses from the
neuron became more frequent and more intense.
[E.Fetz and M.A.Baker, J.NEUROPHYSIOLOGY, Vol.36,
p179-204 (1973)]
A form of short-term memory referred to as working memory is associated with the ability to store contemporary representations of the outside world. The apparent locus for working memory is in the prefrontal area of the cerebral cortex. Monkeys shown that food is hidden behind an obscuring object, but who are restrained from immediately taking the food, maintain knowledge of the food behind the obscuring object. The monkeys will take the food after a delay, when restraints have been removed. Monkeys with areas of their prefrontal cortex surgically removed will forget about the food immediately after they can no longer see it ("out of sight, out of mind"). Dopamine seems to be the most important neurotransmitter for neurons involved in working memory.
Chronic alcoholics show learning-deficit symptoms of "Korsakoff's syndrome" as a consequence of damage to the mammillary bodies of the hypothalamus and the dorsomedial nucleus of the thalamus. Such patients have difficulty discriminating objects and words. Acquisition of new knowledge based on discrimination is very slow and short-term memory is poor, but long-term memory is not so impaired.
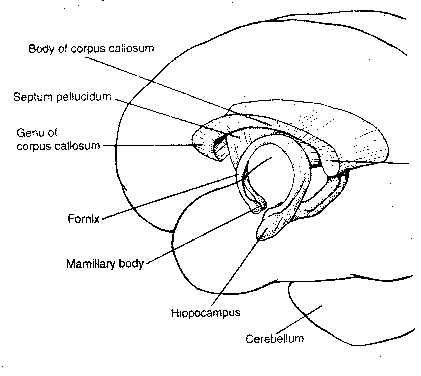
|
The term "limbic system" is an umbrella
term to describe the cingulate gyrus, the
hippocampus, the septal nuclei and
the amygdaloid nuclei. Sometimes the
mammillary nuclei and anterior thalamic
nuclei are also included. Although the
limbic structures have been described as
the "emotional brain", the functional
unity of these structures remains to be
proven. The cingulate gyrus seems to be
the channel through which the cerebral cortex
influences autonomic functions like
respiration and heart rate (often linked
to emotion). Removal of the cingulate gyrus
from monkeys makes them more tame, but also more
indifferent to social interaction. Lesions to
the cingulate gyrus to humans reduce the
experience of pain. Artificial stimulation of
the amygdala causes animals to demonstrate
signs of strong fear or rage. Monkeys whose amygdala
have been lesioned can learn to recognize objects, but
have difficulty associating objects with reward or
punishment. Lesions to the septal
nuclei reduce aggression and alter sexual behavior.
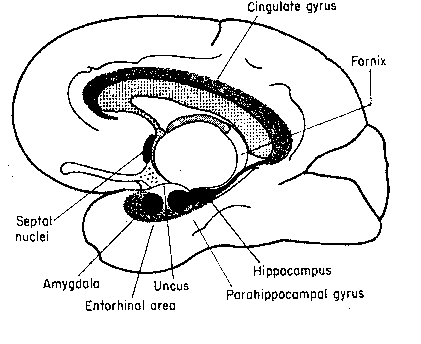
|
In 1953 a 27-year-old patient referred to as
"H.M." was subjected to surgery to relieve extreme
epilepsy. H.M. lost his hippocampus, amygdala and
parts of his parahippocampal gyrus on both sides
of his brain. Thereafter, H.M. lost his ability
to form new memories of facts. He only remembered
facts acquired some time before his operation. He
could acquire new skills, but had no memory of
having acquired them. Nor were these
simply motor skills. By repeated practice,
he improved his ability to solve the
Tower of Hanoi problem. He also learned
to read and form letters through a
mirror (a type of learning reportedly
mediated by the striatum). He could
not recall people whom he met on a
regular basis, but had not known
prior to his operation. Shortly
after having finished a meal, he
could start another one without
remembering he had just eaten. (His
feelings of hunger and satiety were
also diminished.) H.M. once described
his life with the words, "Every day is alone,
regardless of the pleasures I have had or
the sorrows I have had."
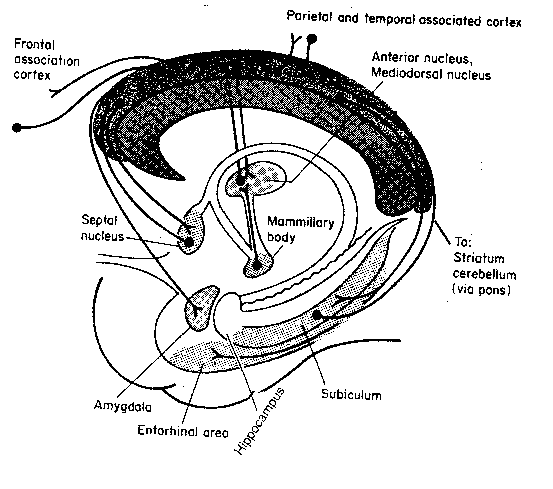
|
H.M. seems the archetypal case of a person who is capable of memory, but not of learning. For a cryonicist, this raises the question, "Is the hippocampus essential for the preservation of identity?". The loss of an eye or a hand would be tragic, but it would not mean loss of identity. A future technology may yet provide us with a new eye or a new hand — or perhaps a new hippocampus.
The hippocampus has proven to be a boon to
neuroscience. Not only does it seem to be a central
organ of learning, but its physiology is very
amenable to laboratory study, thanks to its lamellar
structure. Nonetheless, although many suggestive
correlations are seen between the hippocampal
anatomy&physiology and hippocampal function,
there is not the causal connectedness that is
seen with Aplysia experiments.
| Hippocampus Structure | Hippocampus Connections |
|---|---|
![[ Hippocampus Structure ]](./FigIII24.gif)
|
![[ Hippocampus Connections ]](./FigIII25.gif)
|
The hippocampus is distinguished by 3 distinctive regions composed of 3 distinctive kinds of cells: the dentate gyrus, which is composed of granule cells and the CA3 and CA1 regions, which are composed of pyramidal cells having different properties. A rough description of the pathway of signals through the hippocampus would be: from the entorhinal cortex to the dentate region, from the dentate gyrus to the CA3 region, from the CA3 region to the CA1 region, and from the CA1 region back to the entorhinal cortex. Nonetheless, within CA3 and CA1 there is a massive input from collateral fibers. Of the roughly 16,000 synapses seen on a typical CA3 neuron, approximately 12,000 of those synapses will be inputs from other CA3 neurons. CA1 neurons, though also having many internal collaterals, primarily receive input from CA3 neurons. Inputs to the hippocampus from the septal nuclei are neuromodulatory — they bathe groups of neurons in acetycholine, thereby increasing their excitability.
Neurogenesis (proliferation, migration and differentiation of neurons) is not found in the adult mammalian brain except in the olfactory bulb and in the hippocampus. The new neurons are "spawned" from astrocytes with neural stem cell potential [THE JOURNAL OF NEUROSCIENCE; Aberg,MA; 21(18):7153-7160 (2001)]. Neurogenesis in the hippocampus evidently plays a role in both acquisition of new memories and clearance of memories not needed in the hippocampus once the memories have been consolidated in the cerebral cortex. Ethyl alcohol, especially when ingested in a large bolus in "binge drinking", can have a devestating effect on neurogenesis in the hippocampus [PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES (USA); Herrera, DG; 100(13):7919-7924 (2003)]. Cortisol also inhibits hippocampal neurogenesis, but DHEA has a stimulatory effect. Cortisol can actually kill hippocampal neurons, and does so increasingly with aging and stress, but this effect can be reversed with melatonin and Insulin-like Growth Factor (IGF-1) [THE JOURNAL OF NEUROSCIENCE; Aberg,MA; 20(8):2896-2903 (2000)]. Exercise (running) has been shown to increase cell proliferation and neurogenesis in the dentate gyrus of the adult mouse [NATURE NEUROSCIENCE; van Praag,H; 2(3):266-270 (1999)].
Another famous neurological patient, designated "R.B." suffered an ischemic incident during surgery that selectively destroyed the CA1 neurons on both sides of his hippocampus (as later revealed by autopsy). R.B. had difficulty remembering the previous day, and he would tell the same stories repeatedly at short intervals. (In monkeys, destruction of the hippocampus alone does not produce nearly as great a learning deficit as destruction of the hippocampus and the amygdala. There is evidence that the hippocampus is more critical for humans than it is for monkeys.)
All the 3 groups of neurons in the hippocampus exhibit a phenomenon known as long-term potentiation (LTP), although in different ways. In 1973, microelectrodes were applied to the perforant path leading to the dentate gyrus of rabbits, and trains of electrical stimulation were administered at the rate of roughly 15 cycles per second for 10-15 seconds. One or two such exposures was enough to "condition" (potentiate) an increased response to the perforant path for up to 10 hours. It was later discovered that optimal LTP training is at a frequency of 5 cycles per second — the frequency of so-called theta waves. Animals exploring an environment display hippocampal EEG rhythms in the theta range. Rats exposed to an enriched environment show substantial potentiation of the perforant path.
Induction of LTP in the CA1 pyramidal cells increased the number of shaft synapses seen in electron microscopy by one third. The physiology of LTP in the CA1 synapses has been subject to the most research because of the presence of glutamate receptors known as NMDA receptors. Although NMDA receptors are widely distributed in the brain — especially concentrated in the cerebral cortex and basal ganglia — NMDA receptors are particularly concentrated in the CA1 region of the hippocampus. It is worth noting that NMDA receptors are not present in CA3 synapses, and the mechanism of LTP there remains unknown.
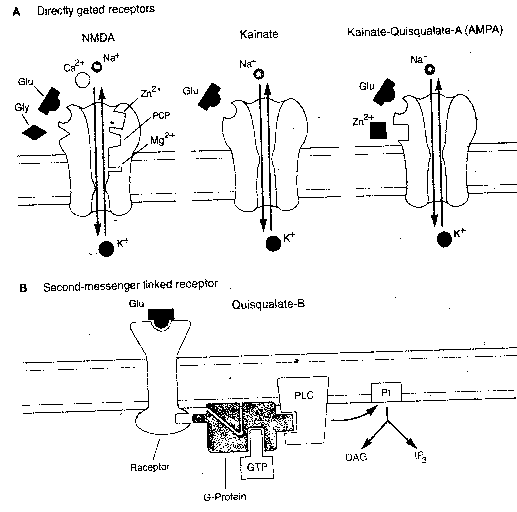
At least four types of glutamate
receptors can be distinguished in
the brain. Two glutamate receptors
are directly gated and control ion
channels that pass sodium (Na+) and
potassium (K+) ions. NMDA receptors
(so-called because they are selectively
stimulated by N-Methyl-D-Aspartate)
are directly gated and control ion
channels that pass Na+, K+ and Ca2+
(calcium ions) as well. Moreover,
NMDA receptors are normally blocked by
Mg2+ (magnesium ion) and do not function
effectively unless they are bound by
glycine. NMDA receptors are also
distinguished from other glutamate
receptors by the fact that they are
selectively blocked by the drug APV
and are inhibited by the hallucinogenic
drug PCP ("angel dust"). The fact that NMDA
receptors are directly gated, but pass calcium
ion, allows them to induce a second-messenger
system that is not mediated by G-protein
(probably Ca2+/Calmodulin Kinase) resulting in
long-term structural changes to the
post-synaptic cell. Moreover, the activation
of kinase is thought to induce synthesis of
the molecule nitric oxide (NO), which is so
small that it effortlessly passes through cell
membranes — acting as a "retrograde second
messenger" to induce the presynaptic neuron
to enhance transmitter release.
|
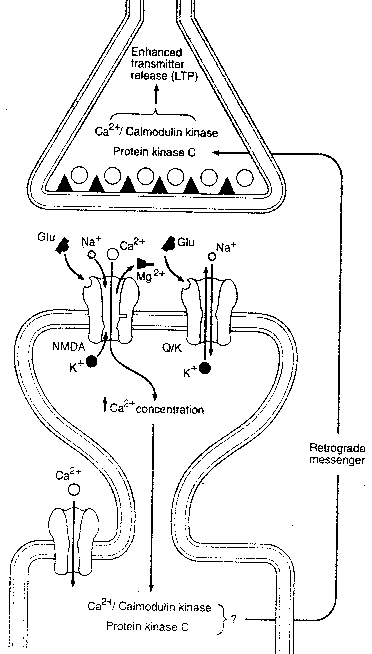
|
CA1 postsynaptic membranes contain NMDA glutamate receptors as well as non-NMDA (directly-gated) glutamate receptors. During normal low-frequency stimulation, only the non-NMDA channels will open, due to Mg2+ blockage of the NMDA ion channels. But high- frequency presynaptic input resulting in depolarization of the postsynaptic membrane displaces the magnesium ions, making the NMDA receptor sensitive to subsequent release of glutamate neurotransmitter.
It seems plausible that LTP mechanisms allow for a kind of learning involving a single episode, rather than repeated trials. It is speculated that associations formed in the CA3 matrix of neurons are built into more economical form in the CA1 neurons. And, in fact, the hippocampus itself probably functions by building more economical storage (long-term memory) in the cerebral cortex. Inputs to the entorhinal cortex (and on to the hippocampus) originate only in so-called multi-modal association areas, which integrate information from several sensory modalities. Outputs from the entorhinal cortex are similar multi-modal association areas. In this view, the hippocampus functions as a transient intermediary in the formation of long-term memories in the association areas of the cerebral cortex.